Duration 2:00
Molar Mass / Molecular Weight of Na2CO3 • 10H2O : Sodium carbonate decahydrate
Published 10 Oct 2018
Explanation of how to find the molar mass of Na2CO3 • 10H2O: Sodium carbonate decahydrate. A few things to consider when finding the molar mass for Na2CO3 • 10H2O: - make sure you have the correct chemical formula. - always include the units for molecular weight (grams/mole). - make sure you do the math right - follow the order of operations. Watch: Molar Mass in Three Easy Steps: /watch/YjxW8OBMM3oMW Note that molecular weight, molar mass, and gram formula mass are essentially the same concept. Periodic Table Image from: https://commons.wikimedia.org/wiki/File: Periodic-table.jpg Mole & Stoichiometry Playlist: /playlist/PLZR1BGWBaZ1zrlxkYJDNW_HWdUib0Q8Pa Finding the Molar Mass (sometimes called Molecular Weight although the units are different) of a compound is a essential skill for the chemistry topic of stoichiometry and the first step in converting from moles to grams (or grams to moles). For more chemistry help visit http://www.Breslyn.org .
Category
Show more
Comments - 44






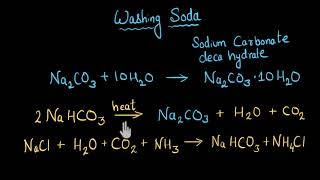




![Washing Soda [Sodium Carbonate Decahydrate] Class-X by Deepanjali Choudhary](https://i.ytimg.com/vi/aRy8z5YzUEk/mqdefault.jpg)






